RESEARCH
Our vision
Animals display a remarkable array of complex adaptive behaviors. They are able to learn from experience and, based on it, make new inferences and flexibly guide decisions in response to changing environmental demands. These behaviors are supported by the finely tuned dynamics of neuronal ensembles distributed across brain circuits. We seek to understand the algorithmic and mechanistic underpinnings of these complex behaviors at the computational, circuit, and cellular levels.
Because tackling these questions requires multiple complementary approaches and novel methods, we develop and implement sophisticated electrophysiological, optogenetic, imaging and computational techniques. Drawing from both computational theories of learning and ethology, we implement novel spatial and social naturalistic behavioral paradigms with a variety of rodent species. We use large-scale electrophysiology and imaging to record hundreds to thousands of neurons across multiple brain areas during behavior. To test the role of specific cells types and brain patterns we perform closed-loop optogenetic manipulations of neural activity, allowing refinement and updating of the theories guiding experiment. In doing so, our experiments inform new theories and models of neural mechanisms of behavior, allowing a continual dialogue between experiment and theory.
Research topics

2- Flexible social behaviors
Social behaviors are a key aspect of many animal species, including rodents and humans. In species that live in groups, the ability to recognize a conspecific and remember previous interactions is an essential part of adaptive behavior. Animals navigate their “social space” by learning hierarchical structures, keeping track of interactions with different emotional valence, establishing and defending territories for survival, and more. To understand the cellular mechanisms of social behaviors it is crucial to perform well-controlled physiological studies in naturalistic conditions. A good animal model for tackling these questions are rodents, since they are social animals and amenable to behavioral assays and neural circuit interrogations.
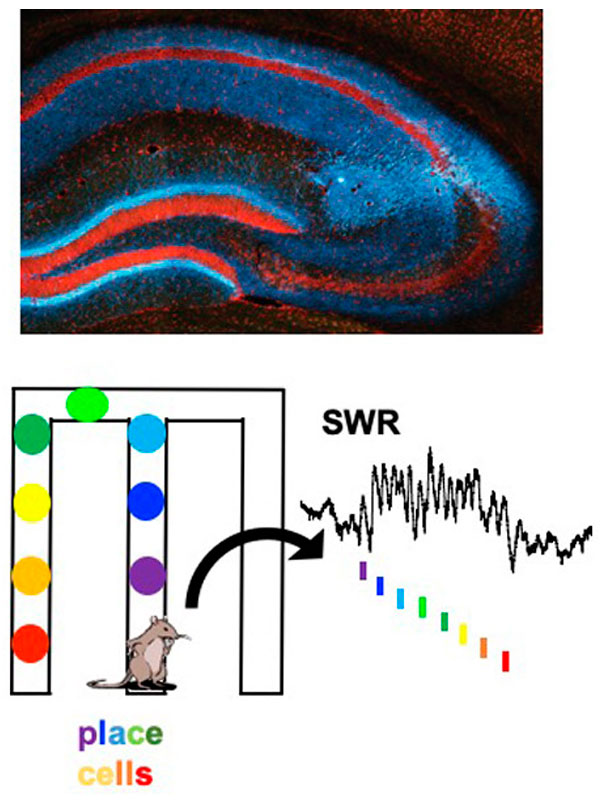
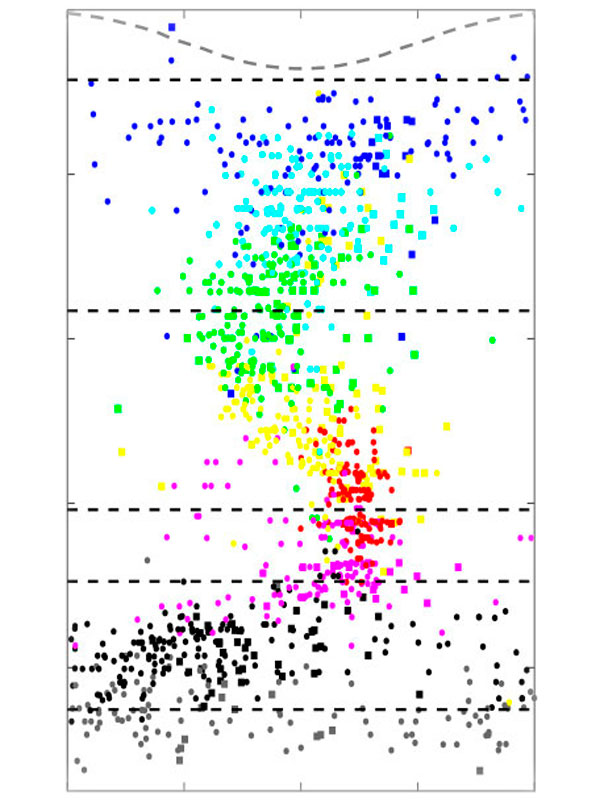
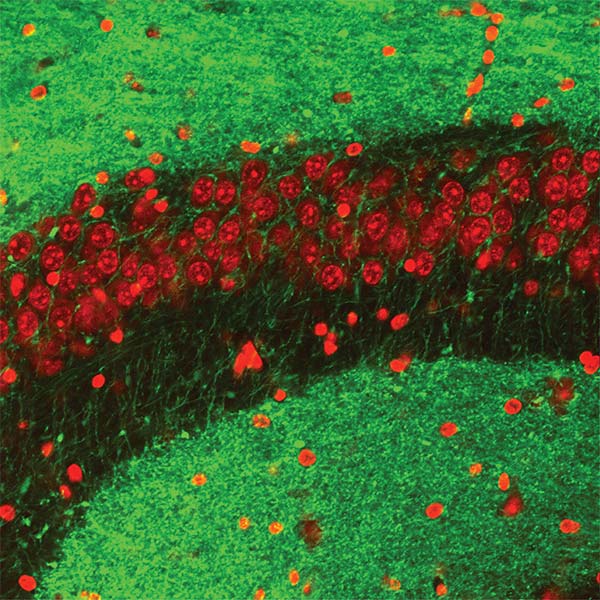
We focus on the hippocampus, where we found cells that encode conspecific identity and form social memory traces that reactivate during SWRs. This area receives strong neuromodulatory inputs from subcortical structures. Neuromodulators, such as serotonin and acetylcholine, regulate both social behaviors and memory processes. Their fluctuations correlate with brain state transitions and enhance the emotional relevance of salient information, potentially through the induction of synaptic plasticity. We investigate how hippocampal-subcortical interactions support social cognition in ecologically relevant conditions.
Techniques
We employ a multi-disciplinary approach, including the development and application of cutting-edge experimental and computational techniques, to understand the algorithms and mechanisms that support flexible behavior.
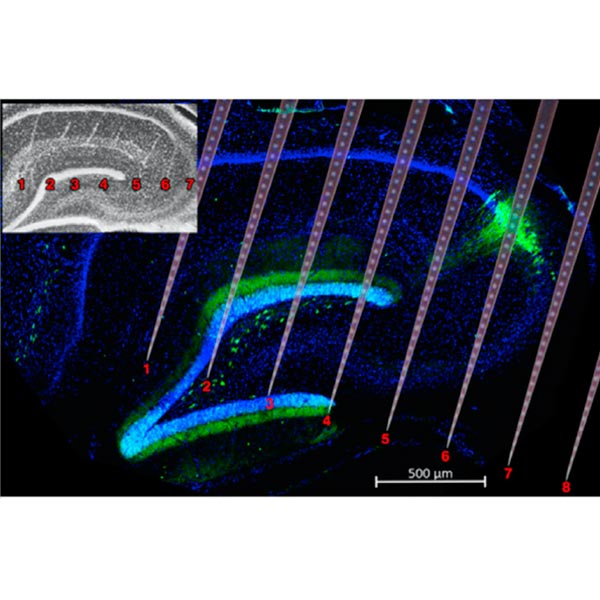
Large-scale electrophysiology
We employ large-scale silicon probes and Neuropixels to record hundreds of single neurons and brain oscillations across multiple brain areas in behaving rodents.

Optogenetics
We use several optogenetic tools to manipulate the activity of genetically defined cell types in order to dissect their contribution to specific behaviors or brain dynamics.
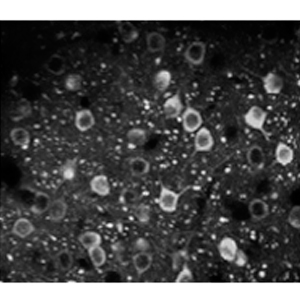
Imaging
Using both multi-photon microscopes or single-photon mini endoscopes, we image the activity of cell ensembles and axons. We also employ fiber-photometry to measure the fluctuations of neuromodulators during behaviors and sleep.

Behavior
Traditional behavioral tasks have been restricted to a few highly simplified paradigms. We develop novel behavioral tasks for complex spatial learning, inference and naturalistic social behaviors.
Data analysis and theory
Our experiments are guided by theory and computational models. We are also constantly developing and implementing novel analysis methods for large-scale neural datasets and the measure and control of behaviors.