PUBLICATIONS
Featured publications
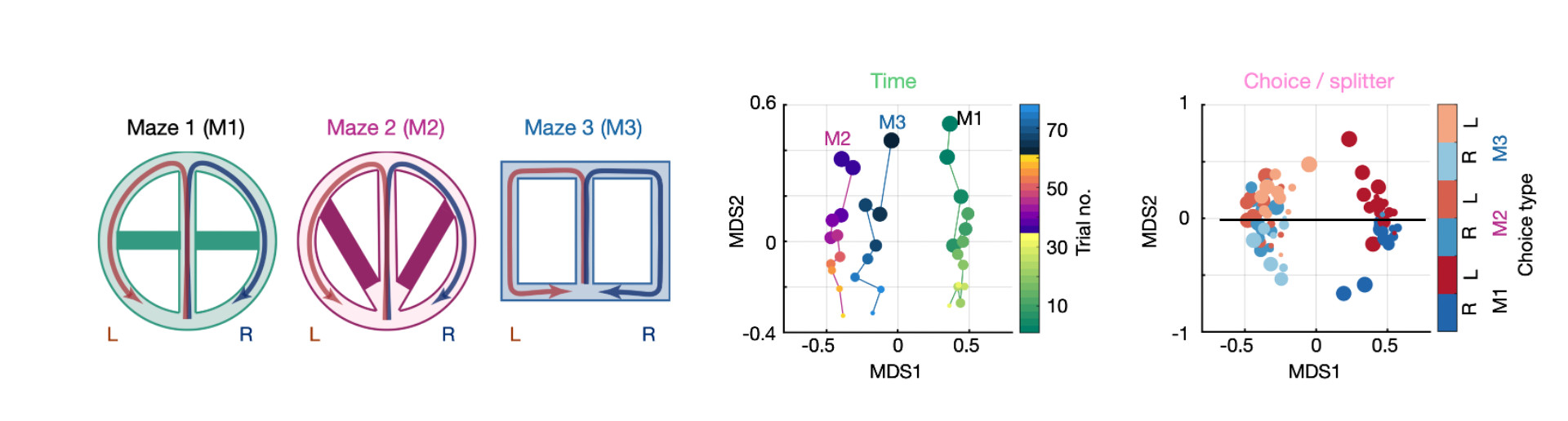
A hippocampal population code for rapid generalization.
Tang W*, Chang H*, Liu C, Perez-Hernandez S, Zheng WY, Park J, Oliva A, Fernandez-Ruiz A. (2025), bioRxiv preprint doi: https://doi.org/10.1101/2025.03.15.643456; link – PDF
Generalizing from experience and applying prior knowledge to new situations is essential for intelligent behavior. Traditional models attribute this generalization to gradual statistical learning in the neocortex. However, such a slow process cannot account for animals’ rapid generalization from limited experience. Here, we demonstrate that the hippocampus supports rapid generalization in mice by generating disentangled memory representations, where different aspects of experience are encoded independently. This code enabled the transfer of prior knowledge to solve new tasks. We identify specific circuit mechanisms underlying this rapid generalization. We show that the seemingly random changes in individual neuronal activity over time and across environments result from structured circuit-level processes, governed by the dynamics of local inhibition and cross-regional cell assemblies, respectively. Our findings provide computational and mechanistic insights into how the geometric structure and underlying circuit organization of hippocampal population dynamics facilitate both memory discrimination and generalization, enabling efficient and flexible learning.
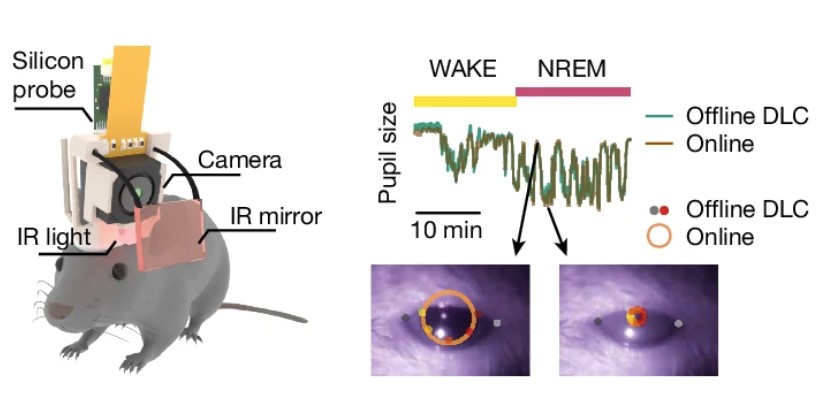
Sleep microstructure organizes memory replay.
Chang H*, Tang W*, Wulf AM, Nyasulu T, Wolf ME, Fernandez-Ruiz A, Oliva A. (2025), Nature, 637, 1161–1169 link – PDF
Recently acquired memories are reactivated in the hippocampus during sleep, an initial step for their consolidation. This process is concomitant with the hippocampal reactivation of previous memories, posing the problem of how to prevent interference between older and recent, initially labile, memory traces. Theoretical work has suggested that consolidating multiple memories while minimizing interference can be achieved by randomly interleaving their reactivation. An alternative is that a temporal microstructure of sleep can promote the reactivation of different types of memories during specific substates. Here, to test these two hypotheses, we developed a method to simultaneously record large hippocampal ensembles and monitor sleep dynamics through pupillometry in naturally sleeping mice. Oscillatory pupil fluctuations revealed a previously unknown microstructure of non-REM sleep-associated memory processes. We found that memory replay of recent experiences dominated in sharp-wave ripples during contracted pupil substates of non-REM sleep, whereas replay of previous memories preferentially occurred during dilated pupil substates. Selective closed-loop disruption of sharp-wave ripples during contracted pupil non-REM sleep impaired the recall of recent memories, whereas the same manipulation during dilated pupil substates had no behavioural effect. Stronger extrinsic excitatory inputs characterized the contracted pupil substate, whereas higher recruitment of local inhibition was prominent during dilated pupil substates. Thus, the microstructure of non-REM sleep organizes memory replay, with previous versus new memories being temporally segregated in different substates and supported by local and input-driven mechanisms, respectively. Our results suggest that the brain can multiplex distinct cognitive processes during sleep to facilitate continuous learning without interference.
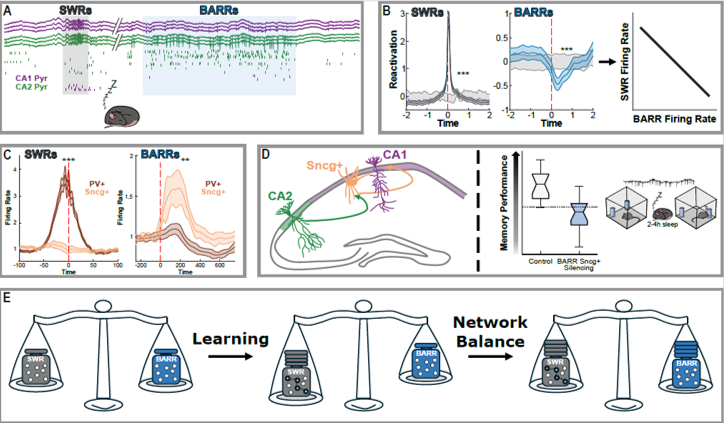
A hippocampal circuit mechanism to balance memory reactivation during sleep.
Karaba LA*, Robinson HL*, Harvey RE, Chen W, Fernández-Ruiz A, Oliva A (2024). Science 385, 6710, link – PDF
Memory consolidation involves the synchronous reactivation of hippocampal cells active during recent experience in sleep sharp-wave ripples (SWRs). How this increase in firing rates and synchrony following learning is counterbalanced to preserve network stability is not understood. We discovered a network event generated by an intrahippocampal circuit formed by a subset of CA2 pyramidal cells to cholecystokinin (CCK+) expressing basket cells, which fire a barrage of action potentials (‘BARR’) during non-REM sleep. CA1 neurons and assemblies that increased their activity during learning were reactivated during SWRs but inhibited during BARRs. The initial increase in reactivation during SWRs returned to baseline through sleep. This trend was abolished by silencing CCK+ basket cells during BARRs, resulting in higher synchrony of CA1 assemblies and impaired memory consolidation.
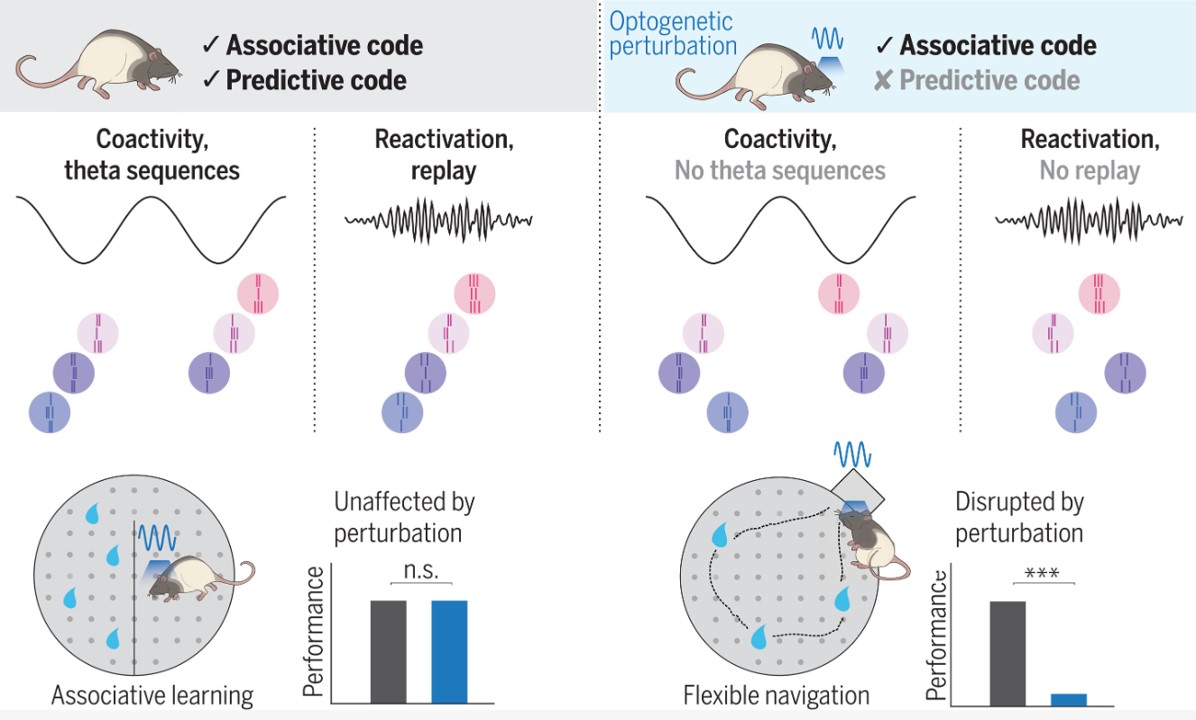
Associative and predictive hippocampal codes support memory-guided behaviors.
Liu C*, Todorova R*, Tang W, Oliva A, Fernández-Ruiz A (2023). Science 382, 6668, link – PDF
Synchronous hippocampal neuronal ensemble activity supports episodic memory. This observation has led to the view that the main function of the hippocampus is to encode associations among different elements of an experience. However, an alternative hypothesis is that the hippocampus generates predictive representations of the world that can guide flexible behaviors. Liu et al. disrupted input from the entorhinal cortex to hippocampal area CA1 (see the Perspective by Steudler and Ólafsdóttir), thus destroying the sequence dynamics of place cells while keeping their coincidental firing intact. Sequence replay was disrupted but assembly reactivations were preserved. Different CA1 codes thus serve corresponding memory operations, with the place code supporting associative memory tasks and the sequence code supporting tasks that require learning about predictive transitions in space.
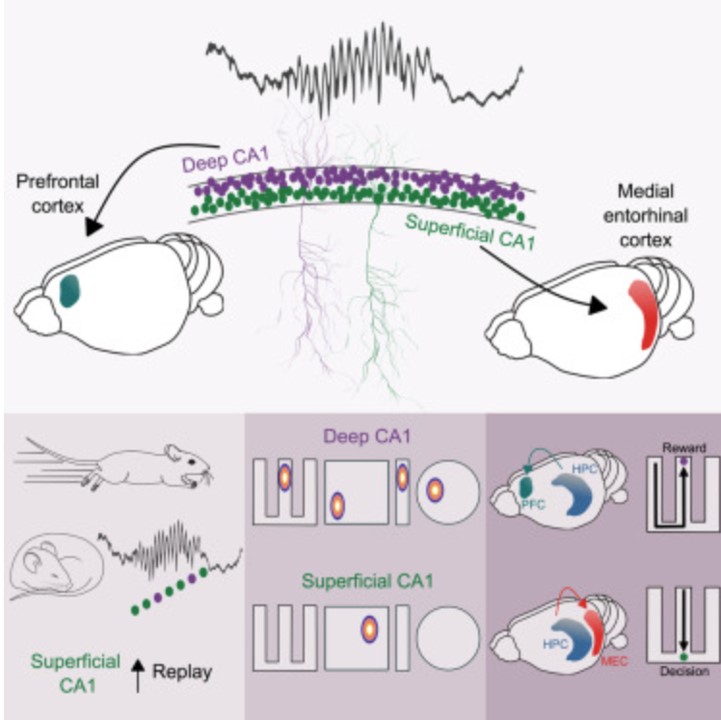
Hippocampo-cortical circuits for selective memory encoding, routing, and replay.
Harvey RE, Robinson HL, Liu C, Oliva A, Fernández-Ruiz A (2023). Neuron 111, 13, P2076-2090, link
Traditionally considered a homogeneous cell type, hippocampal pyramidal cells have been recently shown to be highly diverse. However, how this cellular diversity relates to the different hippocampal network computations that support memory-guided behavior is not yet known. We show that the anatomical identity of pyramidal cells is a major organizing principle of CA1 assembly dynamics, the emergence of memory replay, and cortical projection patterns in rats. Segregated pyramidal cell subpopulations encoded trajectory and choice-specific information or tracked changes in reward configuration respectively, and their activity was selectively read out by different cortical targets. Furthermore, distinct hippocampo-cortical assemblies coordinated the reactivation of complementary memory representations. These findings reveal the existence of specialized hippocampo-cortical subcircuits and provide a cellular mechanism that supports the computational flexibility and memory capacities of these structures.
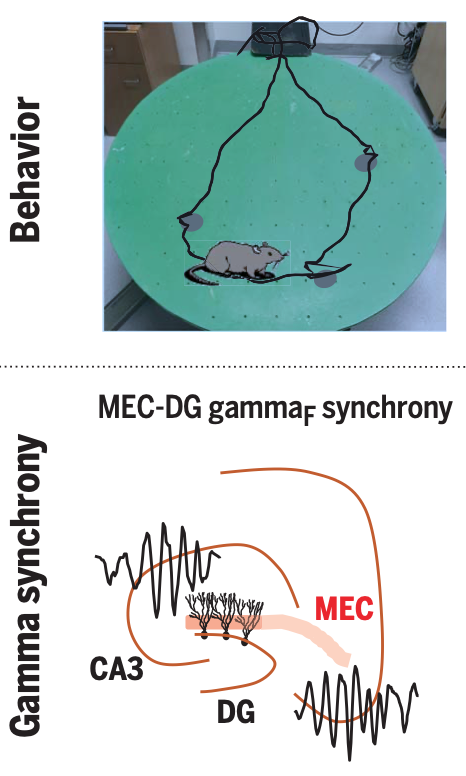
Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies.
Fernández-Ruiz A, Oliva A, Soula M, Rocha-Almeida F, Nagy GA, Martin-Vazquez G, Buzsaki G (2021). Science 372, 6537, link – PDF
Learning induces a dynamic reorganization of brain circuits but the neuro- nal mechanisms underlying this process are not well understood. Interregional gamma- frequency oscillations (~30 to 150 Hz) have been postulated as a mechanism to precisely coordinate upstream and downstream neu- ronal ensembles, for example, in the hippo- campal system. The lateral (LEC) and medial (MEC) entorhinal cortex receive inputs from two distinct streams of cortical hierarchy (the “what” and the “where” pathways) and convey these neuronal messages to the hippocam- pus. However, the mechanisms by which such messages are packaged and integrated or seg- regated by hippocampal circuits had yet to be explored.
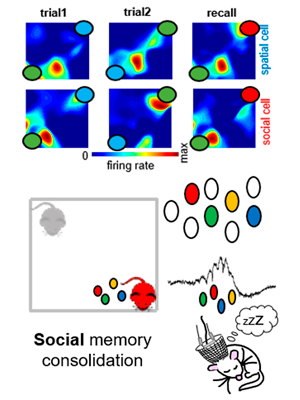
Hippocampal CA2 sharp-wave ripples reactivate and promote social memory.
Oliva A, Fernández-Ruiz A, Leroy F, Siegelbaum S. (2020). Nature 587, 264-269. link – PDF
The hippocampal mechanisms underlying spatial memory are thoroughly studied. These mechanisms involve the reactivation of place cells that had been previously active during recently experienced trajectories. However, how the hippocampus supports higher order, more complex types of memory is yet unclear. Here, we investigated the underlying hippocampal mechanisms of social memory, a higher order form of memory relevant for an animal’s ecological behavior. We found that hippocampal ensembles encoded information related to the identity of different conspecifics, and that such ensembles were highly reactivated during SWRs. Closed-loop disruption of SWRs impaired social memory consolidation. Furthermore, optogenetic generation of ripples from the CA2 region prolong social memory recall. Our findings suggest that SWRs are privileged windows that consolidate multimodal information into a single, relevant representation for an animal’s behavior.
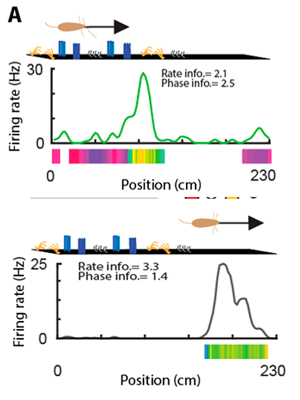
Subcircuits of deep and superficial CA1 place cells support efficient place coding across heterogeneous environments.
Sharif F, Tayebi B, Buzsáki G, Royer S, Fernández-Ruiz A. (2020). Neuron, (in press). link – PDF
The hippocampus is thought to guide navigation by forming a cognitive map of space. However, the behavioral demands for such a map can vary depending on particular features of a given environment. For example, an environment rich in cues may require a finer resolution map than an open space. It is unclear how the hippocampal cognitive map adjusts to meet these distinct behavioral demands. To address this issue, we examined the spatial coding characteristics of hippocampal neurons in mice and rats navigating different environments. We found that CA1 place cells located in the superficial sublayer were more active in cue-poor environments, and preferentially used a firing rate code driven by intra-hippocampal inputs. In contrast, place cells located in the deep sublayer were more active in cue-rich environments and expressed a phase code driven by entorhinal inputs. Switching between these two spatial coding modes was supported by the interaction between excitatory gamma inputs and local inhibition. These findings suggest that environmental features influence the anatomical and computational substrate of hippocampal representation of space.
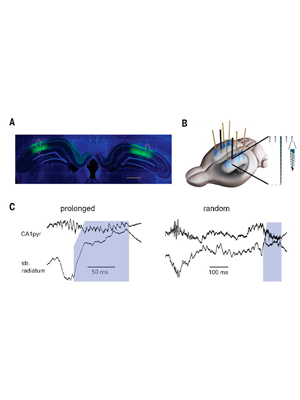
Long-duration Hippocampal Sharp Wave Ripples Improve Memory.
Fernández-Ruiz A*, Oliva A*, Fermino de Oliveira E, Rocha-Almeida F, Tinlgey D, Buzsáki G. (2019). Science 364 (6445), 1082-1086. (* equal contribution) – Link – PDF
Hippocampal sharp wave ripples (SPW-Rs) have been hypothesized as a mechanism for memory consolidation and action planning. The duration of ripples shows a skewed distribution with a minority of long-duration events. We discovered that long-duration ripples are increased in situations demanding memory in rats. Prolongation of spontaneously occurring ripples by optogenetic stimulation, but not randomly induced ripples, increased memory during maze learning. The neuronal content of randomly induced ripples was similar to short-duration spontaneous ripples and contained little spatial information. The spike content of the optogenetically prolonged ripples was biased by the ongoing, naturally initiated neuronal sequences. Prolonged ripples recruited new neurons that represented either arm of the maze. Long-duration hippocampal SPW-Rs replaying large parts of planned routes are critical for memory.
Press coverage:
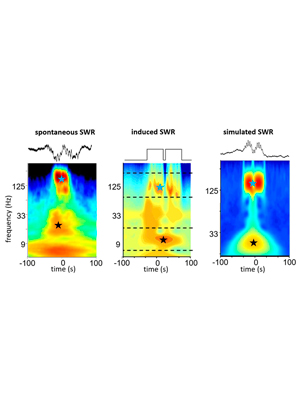
Origin of gamma frequency power during hippocampal sharp-wave ripples.
Oliva A, Fernández-Ruiz A, Fermino de Oliveira E, Buzsáki G. (2018). Cell Reports, 25 (7), 1693-1700. – Link – PDF
Hippocampal sharp-wave ripples (SPW-Rs) support consolidation of recently acquired episodic memories and planning future actions by generating ordered neuronal sequences of previous or future experiences. SPW-Rs are characterized by several spectral components: a slow sharp-wave, a high-frequency “ripple” oscillation, and a slow “gamma” oscillation (20–40 Hz). Using laminar hippocampal recordings and optogenetic manipulations, we dissected the origin of these spectral components. We show that increased power in the 20–40 Hz band does not reflect an entrainment of CA1 and CA3 neurons at gamma frequency but the power envelope of overlapping ripples. Thus, fusion of SPW-Rs leads to lengthening of their duration associated with increased power in the slow gamma band without the presence of true oscillation. These longer SPW-Rs are preceded by increased firing in the entorhinal cortex suggesting a specific generation mechanism.
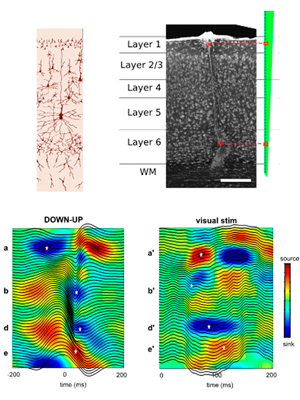
Layer-specific physiological features and interlaminar interactions in the primary visual cortex of the mouse.
Senzai Y*, Fernández-Ruiz A*, Buzsáki G. (2019) Neuron 101,1-14. (* equal contribution) – Link – PDF
The relationship between mesoscopic local field potentials (LFPs) and single-neuron firing in the multi-layered neocortex is poorly understood. Simultaneous recordings from all layers in the primary visual cortex (V1) of the behaving mouse revealed functionally defined layers in V1. The depth of maximum spike power and sink-source distributions of LFPs provided consistent laminar landmarks across animals. Coherence of gamma oscillations (30–100 Hz) and spike-LFP coupling identified six physiological layers and further sublayers. Firing rates, burstiness, and other electrophysiological features of neurons displayed unique layer and brain state dependence. Spike transmission strength from layer 2/3 cells to layer 5 pyramidal cells and interneurons was stronger during waking compared with non-REM sleep but stronger during non-REM sleep among deep-layer excitatory neurons. A subset of deep-layer neurons was active exclusively in the DOWN state of non-REM sleep. These results bridge mesoscopic LFPs and single-neuron interactions with laminar structure in V1.
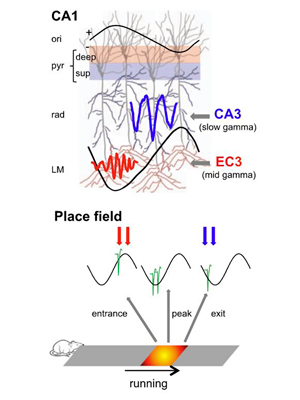
Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling.
Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, Buzsáki G. (2017). Neuron, 93:1213-1226. – Link – PDF
Theta-gamma phase coupling and spike timing within theta oscillations are prominent features of the hippocampus and are often related to navigation and memory. However, the mechanisms that give rise to these relationships are not well understood. Using high spatial resolution electrophysiology, we investigated the influence of CA3 and entorhinal inputs on the timing of CA1 neurons. The theta-phase preference and excitatory strength of the afferent CA3 and entorhinal inputs effectively timed the principal neuron activity, as well as regulated distinct CA1 interneuron populations in multiple tasks and behavioral states. Feedback potentiation of distal dendritic inhibition by CA1 place cells attenuated the excitatory entorhinal input at place field entry, coupled with feedback depression of proximal dendritic and perisomatic inhibition, allowing the CA3 input to gain control toward the exit. Thus, upstream inputs interact with local mechanisms to determine theta-phase timing of hippocampal neurons to support memory and spatial navigation.
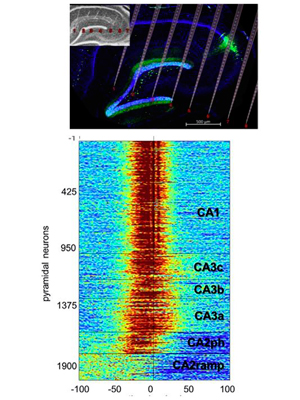
Role of hippocampal CA2 region in triggering sharp-wave ripples.
Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A. (2016). Neuron 91:1342-1355. – Link – PDF
Sharp-wave ripples (SPW-Rs) in the hippocampus are implied in memory consolidation, as shown by observational and interventional experiments. However, the mechanism of their generation remains unclear. Using two-dimensional silicon probe arrays, we investigated the propagation of SPW-Rs across the hippocampal CA1, CA2, and CA3 subregions. Synchronous activation of CA2 ensembles preceded SPW-R-related population activity in CA3 and CA1 regions. Deep CA2 neurons gradually increased their activity prior to ripples and were suppressed during the population bursts of CA3-CA1 neurons (ramping cells). Activity of superficial CA2 cells preceded the activity surge in CA3-CA1 (phasic cells). The trigger role of the CA2 region in SPW-R was more pronounced during waking than sleeping. These results point to the CA2 region as an initiation zone for SPW-Rs.
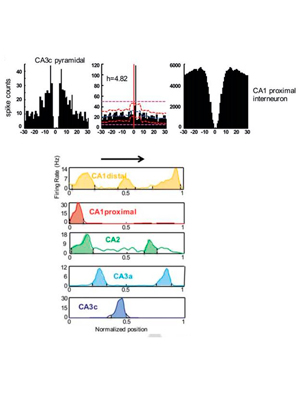
Spatial coding and physiological properties of hippocampal neurons in the Cornu Ammonis subregions.
Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A. (2016) Hippocampus, 26: 1593–1607. – Link – PDF
It is well‐established that the feed‐forward connected main hippocampal areas, CA3, CA2, and CA1 work cooperatively during spatial navigation and memory. These areas are similar in terms of the prevalent types of neurons; however, they display different spatial coding and oscillatory dynamics. Understanding the temporal dynamics of these operations requires simultaneous recordings from these regions. We performed large‐scale silicon probe recordings simultaneously spanning across all layers of CA1, CA2, and CA3 regions in rats during spatial navigation and sleep and compared their behavior‐dependent spiking, oscillatory dynamics and functional connectivity. Our results indicate that a combination of intrinsic properties together with distinct intra‐ and extra‐hippocampal inputs may account for the subregion‐specific modulation of spiking dynamics and spatial tuning of neurons during behavior.
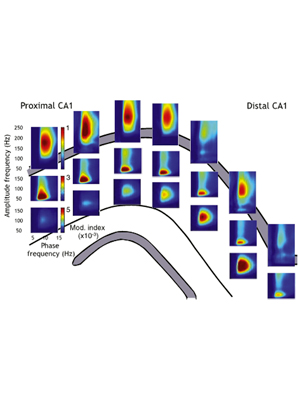
Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks.
Schomburg EW*, Fernández-Ruiz A*, Berényi A, Mizuseki K, Anastassiou CA, Koch C, Buzsáki G. (2014). Neuron. 84:470-485. (* equal contribution) – Link – PDF
Precisely how rhythms support neuronal communication remains obscure. We investigated interregional coordination of gamma oscillations using high-density electrophysiological recordings in the rat hippocampus and entorhinal cortex. In this paper, we characterized for the first time the sources of three distinct gamma oscillation in the hippocampal CA1 area. We found that 30–80 Hz gamma dominated CA1 local field potentials (LFPs) on the descending phase of CA1 theta waves during navigation, with 60–120 Hz gamma at the theta peak. These signals corresponded to CA3 and entorhinal input, respectively. While theta phase coordination of spiking across entorhinal-hippocampal regions depended on memory demands, LFP gamma patterns below 100 Hz in the hippocampus were consistently layer specific and largely reflected afferent activity. Gamma synchronization as a mechanism for interregional communication thus rapidly loses efficacy at higher frequencies.

